
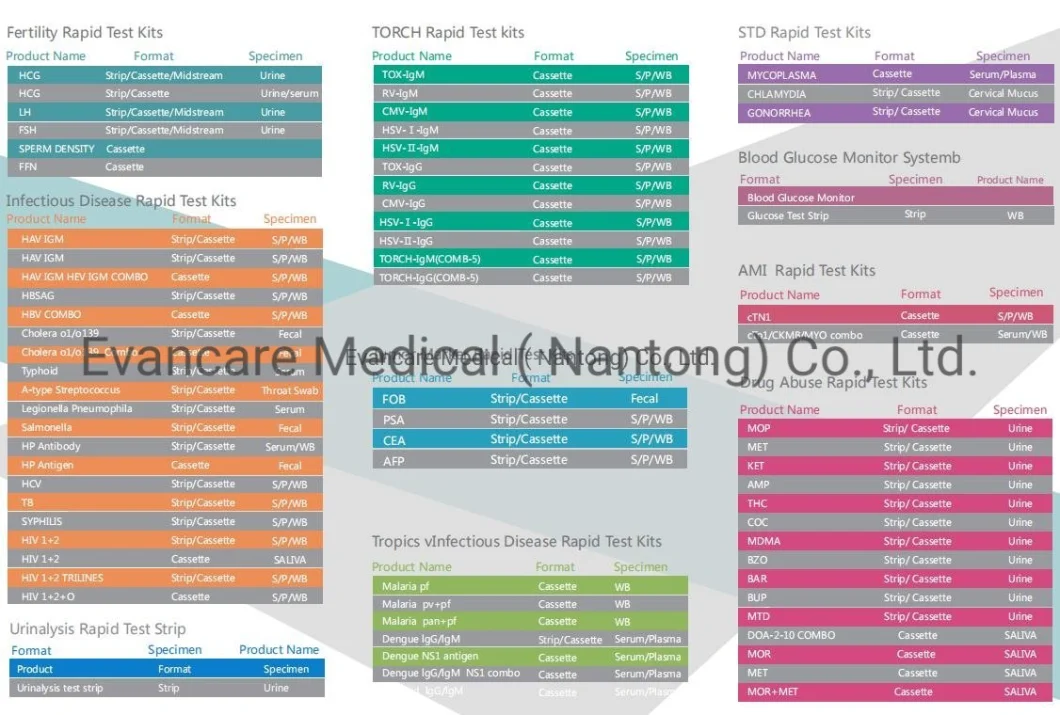
One Step Oncology Cancer Marker Rapid Carcinoembryonic Antigen Test Ctni/Ckmb/Myo Test Kit
Overview Product Description EVANCARE Diagnostic CK-MK Rapid Test Kits For professional and in vitro diagnostic use only
Description
Basic Info
| Model NO. | EC- CK-MB |
| Type | IVD Reagent |
| Certificate | CE, ISO |
| Method | Colloidal Gold Rapid Test |
| Warranty | 3 Years |
| Material | Medical Grade ABS Plastic |
| Package | Bottle + Carton |
| Function | Early Detection |
| Display | Screening |
| Format | Strip+Bottle |
| Name | Ck-MB Test Kit |
| Equipment | by Eye or Machine |
| Reaction Time | 5-10 Seconds |
| Accuracy | 99.7% |
| Delivery Time | 3-5 Days |
| Temperature | 4-30 Degree |
| Storage | Room Temperature |
| Transport Package | Carton |
| Specification | 64*50*44 cm |
| Trademark | evancare or OEM |
| Origin | China |
| HS Code | 3002150090 |
| Production Capacity | 100000PCS/Day |
Product Description
Product Description
EVANCARE Diagnostic CK-MK Rapid Test KitsFor professional and in vitro diagnostic use only.[INTENDED USE]The CK-MB Rapid Test Cassette is a lateral flow immunoassay for the qualitative detection of creatine kinase MB (CK-MB) and its complex in human whole blood, serum or plasma. It provides an aid in the MB diagnosis of acute myocardial infarction (AMI). [PRINCIPLE] CK-MB is an isoform of enzyme creatine kinase with molecular weight 85,745. When the heart cells are damaged, it released to the blood rapidly. The elevated level could be detected approximately as early as 6 hour after onset of AMI. The CK-MB range of normal serum is less than 5 ng/mL. The mean peak level of CK-MB is 21 ng/mL or even higher after the onset of AMI. The CK-MB Rapid Test Cassette is an easy, fast and visually read method that does not require instrumentation, such like ECG. The test system employs unique antibodies, which selectively identifies CK-MB with a high degree of sensitivity. .
Detailed Photos
Positive: *Two lines appear. One colored line should be in the control region (C), and another apparent colored line adjacent should be in the test region (T). Negative: One colored line appears in the control region (C). No line appears in the test region (T). Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test cassette. If the problem persists, discontinue using the lot immediately and contact your local distributor.Item No. | EVANCARE Diagnostic CK-MB Rapid Test Kits |
Contents | Each bag:1 test cassette and 1 desiccant(cassette) 25 cassettes+1 Buffer /box antigen test We can put instruction as your requirement |
Format / Size | Cassette:2.5mm 3.0mm 4.0mm |
Methodology | Sandwich Colloidal Gold |
Assay Type | Qualitative Detection |
Specimen | Antibody (S/P/WB), |
Indicator | CK-MB Test Cassette |
Storage Temperature | 4-30 degree centigrade |
Expiry date | 3 years |
Certificate | CE,ISO,FREE SALE,FDA... |
Detection Limits | Home or Professional Use |
Accuracy | > 99.99% |
| Reaction Time | 3-5 min |
| Delivery | 5-7 days |
| OEM Service | Available |
| Shipping Method | by air or sea or international express |
Company Profile

Certifications

Product Procedure

Activity Exhibition

FAQ
Our Contact
Send now






